Development and Optimization of Temperature Swing Adsorption Cycles
Cyclic adsorption processes have been extensively studied in the past for the purification of the less retained component. This research and development activity has led to commercially available processes such as pressure swing adsorption (PSA) for H2–purification [1], or temperature swing adsorption for gas drying [2]. Recently, many literature studies have emerged regarding promising novel adsorbent materials for CO2 capture from flue gases by TSA [3] as well as structure property relationships as a step towards tailored material design for specific applications [4]. Thermal regeneration is of particular interest due to the possible partial recovery of the sensible heat of the flue gas, which will lower the effective energy consumption of the process [5]. In the case of post-combustion capture, the feed stream is mainly composed of nitrogen and carbon dioxide (CO2), which usually adsorbs more strongly on existing materials [6]. The design of cycles aimed at enriching the heavy product still remains a challenge.
The effective design of these non-stationary processes requires on a deep understanding of the underlying dynamics. The adsorption dynamics can be described by a system of quasi-linear PDE’s which result in non-linear waves, i.e. traveling composition fronts, which can be exploited in efficient separation processes [7]. The cycle design is a crucial aspect for these processes, and must be tailored to the required product specifications and the adsorbent material. The most common approach for studying cyclic adsorption separations and developing cycles is model-based process design [8]. The used mathematical models consist of a system of partial differential equations describing the mass and energy balances of the adsorption bed. These must be numerically solved for every cycle step sequentially until reaching a cyclic steady state, which are generally computationally very intensive.
Equilibrium-based Shortcut Model
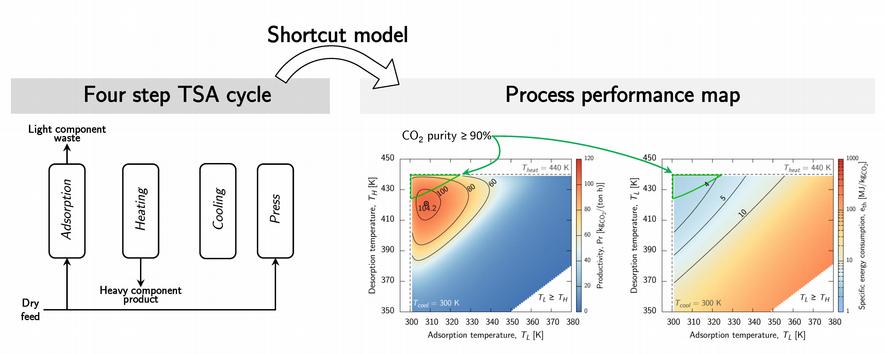
We have developed a shortcut model for a four step TSA cycle aimed at enriching the heavy component [9]. The semi-analytical model takes into account the heat transfer rate, but neglects mass transfer resistances, and computes the cyclic steady state directly. This allows reducing the computation time by a factor of 600 as compared to a standard detailed model.
Thanks to the fast computation, this tool can be of practical interest in the design of TSA processes with regards to the screening of existing adsorbents, and with regards to determining good initial guesses of the operating conditions when performing a multi-objective optimization with a detailed model. Finally, such a shortcut model could be helpful in guiding the development of novel sorbent materials for CO2 capture by TSA following an approach that accounts directly for the key considerations related to the applicability of the materials in the process. In fact, there remains a gap to bridge between the emerging works aiming at determining quantitative structure property relationships of adsorbents and the actual process performance.
Cycle design and optimization of operating conditions
Once a promising cycle, material, and column geometry have been identified, a full optimization of the operating conditions with the detailed model is necessary in order to correctly assess the process performance [10]. This task involves nonlinear multi-objective optimization, where the specific energy requirement must be minimized and the productivity maximized, with constrained purity and recovery.
References
- external page call_made 1. Voss, C. Adsorption 2005, 11, 527–529
- external page call_made 2. Nastaj, J.; Ambrozek, B. Dry. Technol. 2009, 27, 1344–1352
- external page call_made 3. Lin, L.-C.; Berger, A.B.; Martin, R.L.; Kim, J.; Swisher,J.A.; Jariwala, K.; Rycroft, C.H.; Bhown, A.S.; Deem, M.W.; Haranczyk M.; Smit, B. Nat. Mater. 2012, 11, 633–41
- external page call_made 4. Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; Eddaoudi M.; Zaworotko, M.J. Nature 2013, 495, 80-84
- external page call_made 5. Filippi E.; Zardi, F. U.S. Patent 7 153 344, 2002
- external page call_made 6. Wilcox, J.; Haghpanah, R.; Rupp, E.C.; He, J.; Lee, K. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 479–505
- external page call_made 7. Rajendran, A.; Mazzotti, M. Annu. Rev. Chem. Biomol. Eng. 2013, 4, 119-141
- external page call_made 8. Reynolds, S.P.; Mehrotra, A.; Ebner, A.D.; Ritter, J.A. Adsorption 2008, 14, 399–413
- external page call_made 9. Joss, L.; Gazzani, M.; Hefti M.; Marx D.; Mazzotti M. Ind. Eng. Chem. Res. 2015, 54, 3027-3038
- external page call_made 10. Casas, N.; Schell, J.; Joss, L.; Mazzotti, M. Sep. Purif. Technol. 2013, 104, 183-192