Combined CO2 capture and storage by mineralization: Process development
Carbon dioxide (CO2) mineralization belongs to the family of CO2 capture and storage technologies that aims at reducing the greenhouse gas emissions from anthropogenic point sources, thus contributing to combat climate change. Mineralized in the form of thermodynamically stable, environmentally benign carbonates, the CO2 is locked outside the atmosphere for millennia to come. Moreover, such carbonates may prove valuable as substitutes for raw materials in various industries, thus combining the element of storage with the element of utilization.
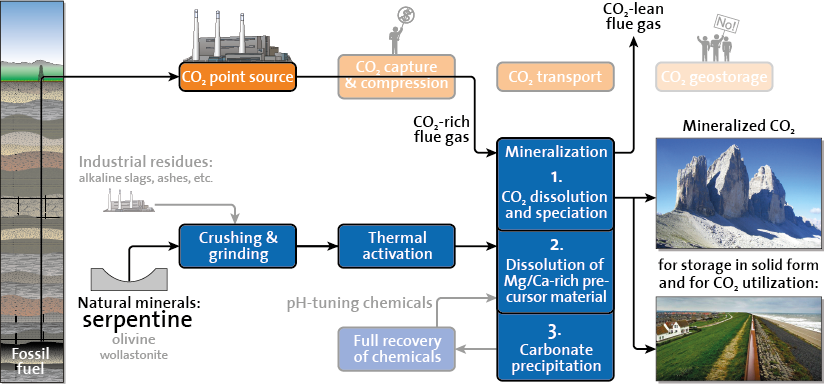
An energy and cost efficient mineralization process needs to fix CO2, at the same time while capturing it from the flue gas. In literature, this challenge is mostly tackled by using pH-tuning agents, which require full recovery, and/or by using precursor materials that are more reactive but much less abundant than natural minerals. At SPL, our approach is to carbonate the Mg-silicate serpentine, the world’s most abundant mineralization precursor that can be thermally activated for enhanced reactivity, in an additive-free aqueous medium at low CO2 pressures, see Fig. 1.
Key thrusts of the project
One of the two key thrusts of our mineralization research project is to investigate the underlying kinetics of the reactions involved, i.e. the dissolution of the precursor material and the precipitation of the carbonate minerals, see Mineralization: Kinetic studies. The insight gained from this work has helped and will help to develop a process that is both technically and economically feasible, which is the second key thrust of our project.
From single- to double-step carbonation
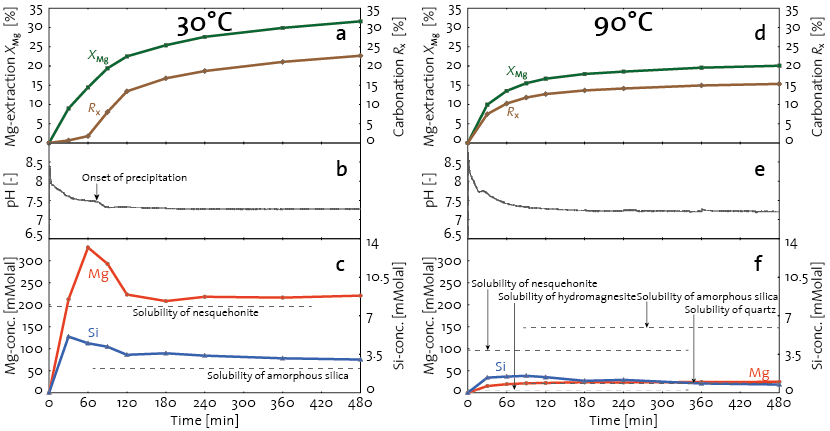
Process investigations started with a single-step carbonation study [1] that was then refined to monitor the solute concentration and the conversion of the Mg in the feed into carbonates at regular intervals during the experiments [2]. This information proved crucial to better understand the precipitation dynamics in the MgO-CO2-H2O system and the fate of the two relevant solutes, i.e. magnesium (Mg) and aqueous silica (Si), see Fig. 2.
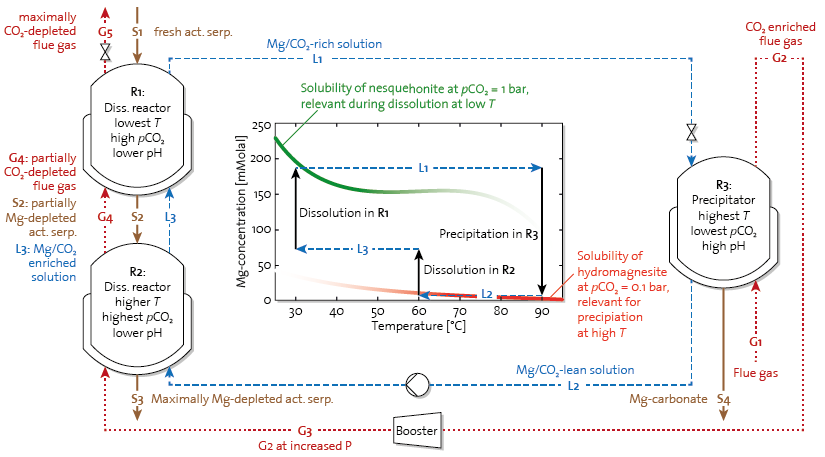
Given the limitations we identified for this operation mode, we tested different strategies to improve the process efficiency [2]. The most promising, i.e. a double-step arrangement where the dissolution step is physically separated from the precipitation step, is currently being further developed. This process concept exploits simple thermodynamics, namely a temperature swing aided by a moderate-level CO2 pressure swing, as illustrated in Fig. 3.
Figure 1: The direct flue gas mineralization value chain, avoiding a separate capture step as well as the unpopular storage of compressed CO2 in an underground geological reservoir. Instead, mineralized CO2 is a stable, environmentally benign solid compound that may serve various industries, e.g. providing construction material for raising the Dutch dams ( …sidenote: this combines climate change mitigation with climate change adaptation).
Figure 2: Two exemplary single-step carbonation experiment at T = 30°C (left column) and 90°C (right column), pCO2 = 1 bar, and S/L = 20% wt.: (a,d) the Mg- and Si-concentration profiles; (b,e) the pH profile (pH(t = 0) ≈ 4, i.e. outside the range of the figure axes); (c,f) the extent of Mg-extraction and carbonation. The dashed lines in (a,b) indicated the solubility levels of the kinetically relevant solid phases at each temperature; for comparison, also the solubility of nesquehonite and amorphous silica are shown in (d).
Figure 3: Flow scheme of a combined T–pCO2-swing process with two dissolution stages and one precipitator, connected by gas streams (dotted lines, red), liquid streams (dashed lines, blue), and solid streams (solid lines, brown). On the left, i.e. the dissolution side, activated serpentine is contacted in counter current flow with the liquid and gas streams in a series of two or more reactors operated at increasing temperatures (w.r.t. the solid stream). On the right, i.e. the precipitation side, Mg-carbonate formation is triggered by the high T and lower pCO2, which cause excess CO2 to degas. The latter is stripped by the incoming flue gas stream, which therefore gets enriched in CO2 before entering the dissolution side. In the center, the underlying driving force of the process can be appreciated from a concentration-temperature diagram showing the Mg solubility concentration of the kinetically relevant Mg-carbonates, along with the evolution of the Mg-concentration resulting from the different stages of a process cycle.