Combined CO2 capture and storage by mineralization: Kinetic studies
A fully functional Carbon Capture and Storage (CCS) scheme has to ensure a completely safe and permanent storage of the captured CO2. Ex situ mineralization enables this by storing CO2 in a chemically stable carbonate form. The goal of this project is to develop an efficient ex situ CO2 mineralization process through a thorough understanding of the various reaction kinetics and thermodynamics involved. In an aqueous mineralization process, Mg ions are leached from (e.g.) serpentine rock and it is then precipitated as carbonates. Thermal activation, through dehydroxylation, accelerates the dissolution kinetics of serpentine. In this project, we investigate and develop first principles models to describe the kinetics of the various steps involved in a CO2 mineralization process that uses dehydroxylated serpentine as the source of magnesium cations. These include
1) Dissolution of dehydroxylated serpentine [1-3]
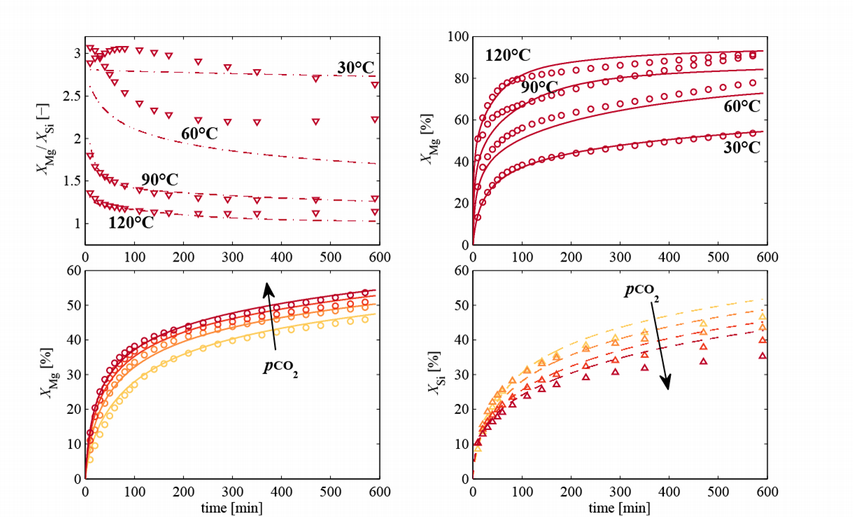
Serpentine dehydroxylation results in significant alteration of the physical morphology and chemical properties of the particles, thus rendering the interpretation of its dissolution profiles non-trivial. The mechanism of dehydroxylation is poorly understood due to the complex nature of its underlying reactions. However, the knowledge of the dissolution kinetics of the silicate, which often is the rate limiting step of the process, is essential to optimize a process design that would allow it to compete with other CO2 sequestration options.
2) Precipitation of magnesium carbonates [4]
The complex nature of the Mg-carbonate system, due to its numerous forms of hydrated and basic carbonates and limited use in industrial applications, has resulted in poor knowledge of the kinetics of its precipitation kinetics. The effective capture rate of CO2 in a mineralization plant is determined by the precipitation kinetics of magnesium carbonates. Of particular interests are the precipitation kinetics of the meta-stable (pseudo) hydromagnesite and the thermodynamically stable magnesite.
Having understood the various reaction kinetics involved, it will be possible to develop a process for CO2 mineralization and to optimize the operational conditions, e.g. the temperature, partial pressure of CO2, slurry density, residence time, etc., see Mineralization: Process development.
References
- external page call_made 1. Werner, M.; Hariharan, S.; Zingaretti, D.; Baciocchi, R.; Mazzotti, M. Chem. Eng. J. 2014, 241, 301-313
- external page call_made 2. Hariharan S.; Werner, M.; Hänchen, M.; Mazzotti, M. Chem. Eng. J. 2014, 241, 314-326
- external page call_made 3. Hariharan S.; Werner, M.; Mazzotti, M. Chem. Eng. J. 2016, 298, 44-54
- external page call_made 4. Hariharan S. and Mazzotti, M. Cryst. Growth Des. 2017, 17 (1), 317-327