Chilled ammonia process for CO2 capture
CO2 capture and storage (CCS) is a bridging technology that can help to achieve the drastic CO2 emission reductions needed to keep global warming within controllable limits – in combination with efficiency measures and a shift towards renewable energy sources [1]. In the CCS value chain, CO2 is first captured from the flue gas of a large point source, before it is compressed and transported to a storage site, where the geological setup is suitable for safe and durable storage in the deep subsurface.
The Chilled Ammonia Process (CAP) is a promising capture technology with a highly competitive energetic performance [2]. The use of aqueous ammonia (NH3) as a solvent offers several advantages compared to the commonly used amine solutions: global availability, low cost, chemical stability also in presence of flue gas impurities such as SOX and NOX, no toxic degradation products.
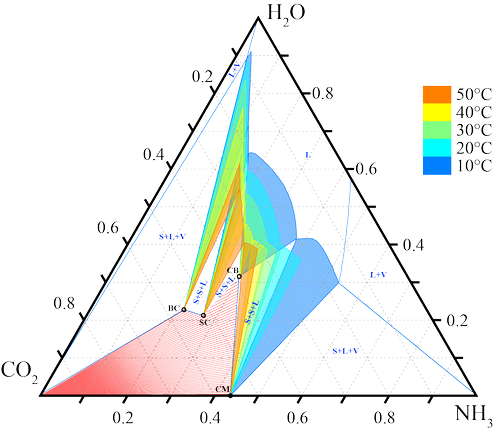
The objective of our research project is to investigate the formation of solids in the Chilled Ammonia Process, i.e. in the CO2-NH3-H2O-system. Four different solid compounds can theoretically form as the CO2 concentration increases along the absorber (see Figure 1). The solid precipitation may lead to clogging of process equipment, e.g. the packed absorber. On the other hand, high CO2 concentrations are beneficial to decrease the heat demand for regeneration of the solvent. The knowledge acquired in our project can be applied to improve the operation of packed absorbers in the CAP by increasing the CO2 loading while avoiding the formation of solids. The research activities include the thermodynamic analysis of the system [3], process synthesis and process simulation [4,5], as well as the experimental investigation of the solid formation in the SPL laboratories.
References
- external page call_made 1. IPCC, Working Group III. Special Report on Carbon Dioxide Capture and Storage. Cambridge University Press, 2005
- external page call_made 2. Gal, E. Patent WO2006/022885A1
- external page call_made 3. Sutter, D.; Gazzani, M.; Mazzotti, M. Chem. Eng. Sci., 2015, 133, 170-180
- Download vertical_align_bottom 4. Sutter, D.; Gazzani, M.; Mazzotti, M. PCCC-2. 2013, Bergen, Norway (PDF, 3.1 MB)
- external page call_made 5. Gazzani, M.; Sutter, D.; Mazzotti, M. Energy Procedia 2014, 63, 1084-1090