Crystal shape engineering
Crystallization is a common technique to separate and purify active pharmaceutical ingredients (APIs) in the pharmaceutical industry. Over 80% of drugs produced undergo a crystallization step at least once. The quality of the resulting product powder is greatly influenced by the crystallization process since properties like processability and bioavailability are closely tied to the particle size and shape distribution (PSSD) of the crystal population. Crystal populations containing needle-like or plate-like shapes, as well as an abundance of fine crystals, are particularly undesirable as they lead to powders with poor flowability and filtrability.
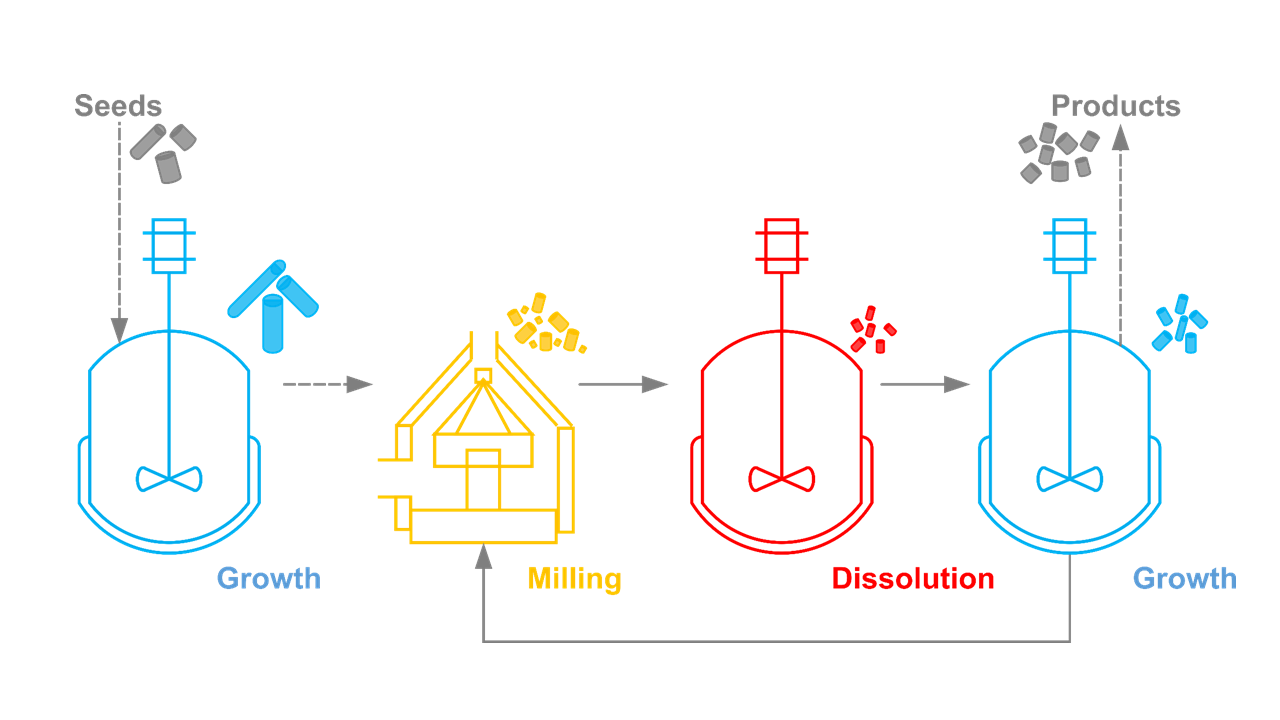
In our research group, we have previously proposed a process to improve the PSSD of the crystals, namely the 3-stage cycle, which is composed of repeated temperature cycling and milling. It transforms needle-like crystals into more equant shapes and dissolves fine crystals, both of which contribute to unfavorable downstream processability. Additionally, feedback control mechanisms have been implemented to enhance the robustness and automation of the process.1,2,3
In order to study shape manipulation processes and effectively control crystal shape, it is essential to have a tool that can monitor the PSSD in real-time. Addressing this requirement, a stereoscopic imaging device capable of measuring up to three characteristic dimensions (length, width, and thickness) of particles has been developed. This device enables characterization of particles with needle-like4 and plate-like shapes5,6, providing valuable insights for shape control studies.
Currently, the effectiveness of existing and novel shape manipulation strategies, such as the 3-stage cycle and the use of additives, is being explored for improving the shape plate-like crystals. Our objective is to further refine and optimize these methods to address the challenges posed by plate-like crystals.
References